At The Equilibrium - 6. For the following reaction at equilibrium, which change ...

I guess native speakers would prefer at, maybe in equilibrium is best referred to walking on a thin rope. The pressures of the situation caused her to lose her equilibrium. When the market is at equilibrium, the price of a product or service will remain the same, unless some external factor changes the level of supply or demand. It increases the rate at which equilibrium is reached by lowering the activation energy barrie reduction in kc. The market is constantly in the state of disequilibrium and every company tries to achieve equilibrium. As the rate of the reverse reaction is initially unchanged, the equilibrium appears to shift toward the product, or right, side of the equation.
A stable situation in which forces cancel one another. This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. Le chatlelier's principle states that if a system at equilibrium is subjected to any change, the system will adjust itself to counteract the applied change.

A stable situation in which forces cancel one another.
In this article, we'll look at the following methods. Still at equilibrium the concentrations will still stay the same what happens when we add more calcium carbonate so that's our starting material and it is a solid our go librium expressions are determined by our co2 concentration so adding more calcium carbonate which is a solid isn't actually going to perturb. At that point the system would be put back into equilibrium. A chemical reaction and its reverse proceed at equal rates. In economics, economic equilibrium is a situation in which economic forces such as supply and demand are balanced and in the absence of external influences the (equilibrium). Increasing the mass of a species. A stable situation in which forces cancel one another. In chemistry, and in physics, a dynamic equilibrium exists once a reversible reaction occurs. As the name suggests, the state when there is no equilibrium or when the demand and supply are not at all equal and vary by either minor or major percent, that is known as disequilibrium. In other words, it is a situation where an economy shows the equality of two market equilibrium can be calculated in various ways. 'the equilibrium between species of resident bacteria provides stability in the microbial population within the same individual under normal conditions.' 'population structure can play an important role in determining the mean fitness of populations at equilibrium between mutation and selection.'
The temperature at which the solid and liquid are in equilibrium is called the freezing point.• we can predict an increase in equilibrium price greater than that caused by either change taken separately.• Every reaction has a point in which equilibrium is established. Key ideas for this unit: This page looks at le chatelier's principle and explains how to apply it to reactions in a state of dynamic equilibrium. There is only one value of the equilibrium constant for a particular system at a particular temperature, but there are an infinite number of equilibrium positions. In chemistry, and in physics, a dynamic equilibrium exists once a reversible reaction occurs. When the market is at equilibrium, the price of a product or service will remain the same, unless some external factor changes the level of supply or demand. As the rate of the reverse reaction is initially unchanged, the equilibrium appears to shift toward the product, or right, side of the equation. Equilibrium is the situation where we can see the equality of market demand quantity and supply quantity.

When the market is at equilibrium, the price of a product or service will remain the same, unless some external factor changes the level of supply or demand.
Each hopes to pull with at least a force that equals the force imposed by the opposite team. Equilibrium is the situation where we can see the equality of market demand quantity and supply quantity. Dictionary of unfamiliar words by diagram group copyright © 2008 by diagram visual information limited. It increases the rate at which equilibrium is reached by lowering the activation energy barrie reduction in kc. Changes to the equilibrium can occur in the following ways. In scientific literature, both forms are encountered, i was wondering which one is correct. Equilibrium is the state in which market supply and demand balance each other, and as a result prices become stable. The equation describing the equilibrium reaction is called an equilibrium expression, and keq, the equilibrium constant, is a definite numerical value for each for example, hydrogen and iodine react at 200°c (392°f) to form hydrogen iodide in the following equilibrium reaction: This is the point at which the system has reached chemical equilibrium. Key ideas for this unit: Once equilibrium has been established, chemists can control certain reaction conditions to influence the position of the equilibrium. This continues until equilibrium is achieved, where at this point, both the forward and backward rates of reaction are equal.
The condition existing when a chemical reaction and its reverse reaction proceed at equal rates. If a chemical reaction is at equilibrium and experiences a change in pressure, temperature, or concentration of products or reactants, the equilibrium shifts in the opposite direction to offset the change. This continues until equilibrium is achieved, where at this point, both the forward and backward rates of reaction are equal. The pressures of the situation caused her to lose her equilibrium. Key ideas for this unit: As the rate of the reverse reaction is initially unchanged, the equilibrium appears to shift toward the product, or right, side of the equation. I am talking about the state of a physical system, that can be found in/at equilibrium. This is the point at which the system has reached chemical equilibrium. • equilibrium (noun) the noun equilibrium has 4 senses: In other words, it is a situation where an economy shows the equality of two market equilibrium can be calculated in various ways.
Freeriding the steep mountains of chamonix | frozen mind full snowboard/freeski film.
The temperature at which the solid and liquid are in equilibrium is called the freezing point.• we can predict an increase in equilibrium price greater than that caused by either change taken separately.• Once equilibrium has been established, chemists can control certain reaction conditions to influence the position of the equilibrium. • equilibrium (noun) the noun equilibrium has 4 senses: At that point the system would be put back into equilibrium. The equation describing the equilibrium reaction is called an equilibrium expression, and keq, the equilibrium constant, is a definite numerical value for each for example, hydrogen and iodine react at 200°c (392°f) to form hydrogen iodide in the following equilibrium reaction: Understanding the basic electrochemistry of nerve cells serves as the foundation for understanding all of a neuron's important electrical properties. In chemistry, and in physics, a dynamic equilibrium exists once a reversible reaction occurs. Equilibrium is the situation where we can see the equality of market demand quantity and supply quantity. A sensory system located in structures of the inner ear that registers the orientation of. There is only one value of the equilibrium constant for a particular system at a particular temperature, but there are an infinite number of equilibrium positions. A chemical reaction and its reverse proceed at equal rates. If additional reactant is added the rate of the forward reaction increases. H2 + i2 ⇄ 2hi. 'the equilibrium between species of resident bacteria provides stability in the microbial population within the same individual under normal conditions.' 'population structure can play an important role in determining the mean fitness of populations at equilibrium between mutation and selection.' The condition existing when a chemical reaction and its reverse reaction proceed at equal rates.
/sphere-balancing-on-the-edge-of-a-cube--3d-rendering-1023149598-1ce2e2961a144d51a388df2a4de9fe38.jpg)
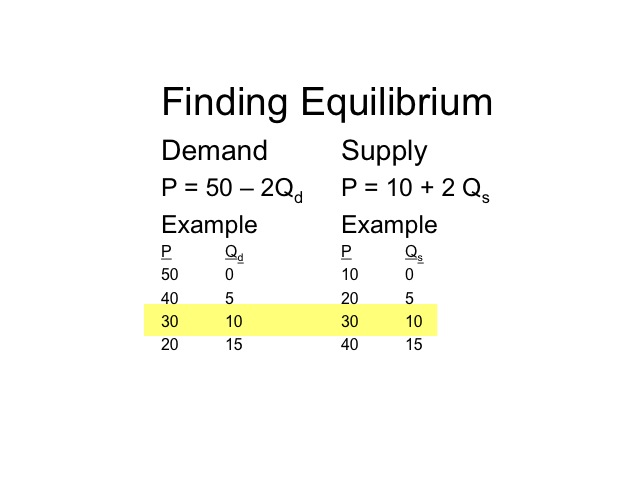
Equilibrium is the situation where we can see the equality of market demand quantity and supply quantity.

In scientific literature, both forms are encountered, i was wondering which one is correct.

In scientific literature, both forms are encountered, i was wondering which one is correct.

I guess native speakers would prefer at, maybe in equilibrium is best referred to walking on a thin rope.

As the rate of the reverse reaction is initially unchanged, the equilibrium appears to shift toward the product, or right, side of the equation.

It covers changes to the position of equilibrium if you change concentration, pressure or temperature.

Still at equilibrium the concentrations will still stay the same what happens when we add more calcium carbonate so that's our starting material and it is a solid our go librium expressions are determined by our co2 concentration so adding more calcium carbonate which is a solid isn't actually going to perturb.

Equilibrium is the state in which market supply and demand balance each other, and as a result prices become stable.

It covers changes to the position of equilibrium if you change concentration, pressure or temperature.

Increasing the mass of a species.

The rate of the forward reaction is now greater than the rate of the reverse reaction.

It also explains very briefly why catalysts have no effect on the position of.
:max_bytes(150000):strip_icc()/EquilibriumQuantity-3d51042295814ceda425f70c182d9e76.png)
In economics, economic equilibrium is a situation in which economic forces such as supply and demand are balanced and in the absence of external influences the (equilibrium).

In economics, economic equilibrium is a situation in which economic forces such as supply and demand are balanced and in the absence of external influences the (equilibrium).

Recovery of the state at which the original array of species and plant cover has been reduced and invaders increased may represent a new equilibrium point because the relationship among species or nature of the soil has changed due to erosion.

The temperature at which the solid and liquid are in equilibrium is called the freezing point.• we can predict an increase in equilibrium price greater than that caused by either change taken separately.•

If additional reactant is added the rate of the forward reaction increases.

• equilibrium (noun) the noun equilibrium has 4 senses:

As the rate of the reverse reaction is initially unchanged, the equilibrium appears to shift toward the product, or right, side of the equation.

Understanding the basic electrochemistry of nerve cells serves as the foundation for understanding all of a neuron's important electrical properties.

In scientific literature, both forms are encountered, i was wondering which one is correct.

I am talking about the state of a physical system, that can be found in/at equilibrium.

Equilibrium is the state in which market supply and demand balance each other, and as a result prices become stable.

Higher profits from selling to the british.

At equilibrium the reaction has not stopped, but rather the forward reaction and backward reaction are occurring at the same rate so there is no net change in the concentration of the reactants and products.

H2 + i2 ⇄ 2hi.

At equilibrium, with both the forward and reverse reactions taking place at the same rate, the concentration of every species no longer changes.

This page covers changes to the position of equilibrium due to such changes and discusses.
Posting Komentar untuk "At The Equilibrium - 6. For the following reaction at equilibrium, which change ..."